Experience a drug-free migraine treatment today
Get back to life with CEFALY: the FDA-cleared migraine treatment device that can relieve pain and give you more migraine-free days. With no prescription needed, this simple-to-use medical device targets the trigeminal nerve to treat acute migraine attacks quickly and prevent future episodes.
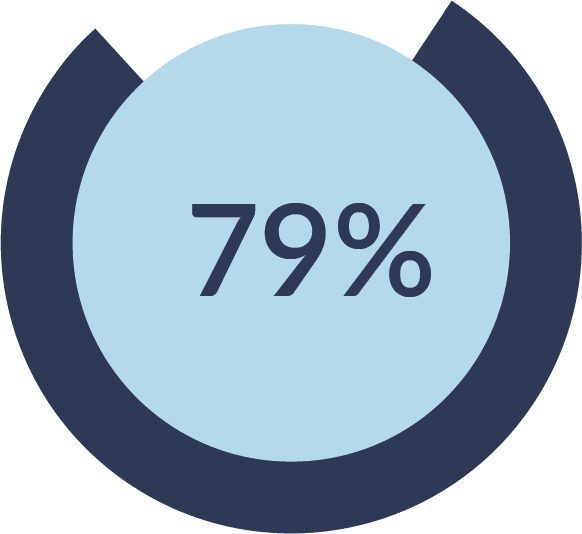
CEFALY is safe and effective, with no serious adverse side effects reported in any clinical study. At last, you can treat migraine without the forgetfulness, grogginess, and other bothersome side effects that come with some medications. Don't let migraine keep you from doing the things you love! Order CEFALY today.

Migraine pain relief and prevention in one device
Worn on the forehead, CEFALY uses a mild electric current (e-TNS) to stimulate and desensitize the trigeminal nerve, the primary pathway for migraine pain.
With CEFALY, you’re in control of your migraine treatment:
Use the ACUTE treatment mode to relieve pain at the first sign of a migraine attack.
Use the PREVENT treatment mode daily to decrease migraine frequency.
Optimize your treatments by tracking sessions on the CeCe app with the Bluetooth-enabled CEFALY Connected.
As seen on


Recommended by healthcare professionals
Featured blogs
Learn about migraine, discover migraine prevention strategies, and get tips for using your CEFALY for maximum migraine relief.
Why Do I Get a Migraine After Exercise —and How Do I Stop It?
Learn more about the link between exercise and migraine and learn how to combat migraine attacks after exercise.
What Does CEFALY Feel Like?
Many people are a little nervous when they try CEFALY for the first time. They wonder: What does the stimulation feel like? Will I be able to tolerate it? Does CEFALY hurt? Learn more.

 FDA-cleared
FDA-cleared Drug-free pain relief
Drug-free pain relief Payment plans available
Payment plans available 90-day money-back guarantee
90-day money-back guarantee